Written by Jonah Udall; Select sections of article written by and article reviewed by Dr. Bojana Jankovic Weatherly.
The vast community of microorganisms that live in our gut are vital to maintaining our optimal health. Altogether, these microbes weigh approximately 2 kilograms, roughly as much as the liver – one of our most metabolically active organs. Indeed, the microbiome may be considered an organ in and of itself, containing more genes than the human body. 1
Changes in this dynamic community may contribute to systemic diseases far beyond the gastrointestinal tract, from fatty liver to arthritis, and rosacea to depression. In the relatively young field of microbiome research, certain microbial molecules have emerged as central players in human health. One of the most important of these is lipopolysaccharide, or “LPS.”
What is lipopolysaccharide (LPS)?
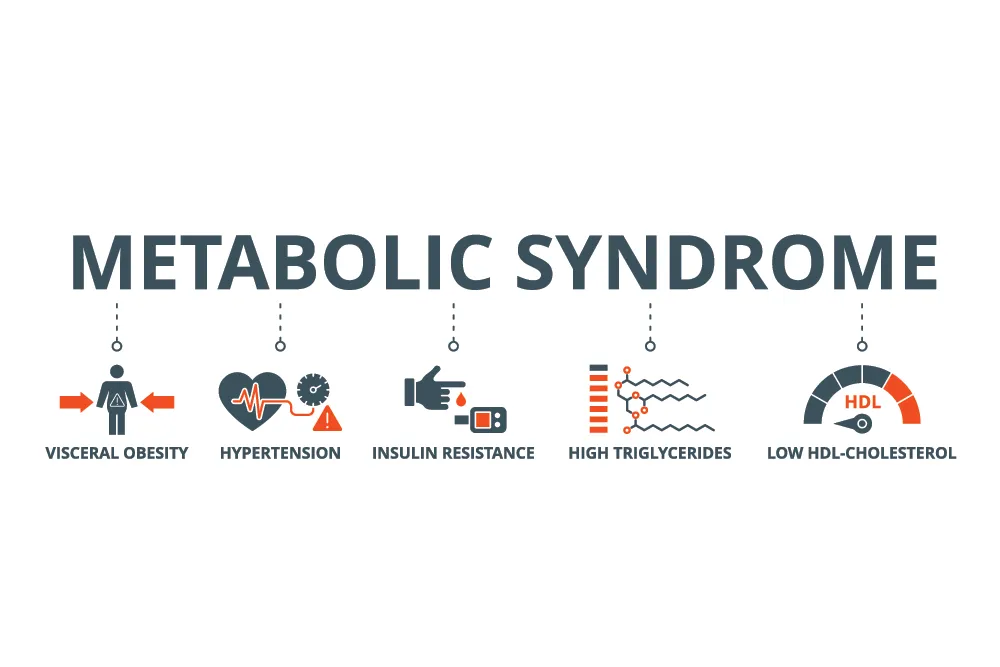
Bacteria are characterized as either Gram-negative or Gram-positive depending on whether their cell wall stains violet when a dye is applied. Gram-positive bacteria have a thick cell wall, which retains the violet stain, but they lack an outer membrane. Conversely, Gram-negative bacteria have a thin cell wall but are surrounded by an outer membrane containing lipopolysaccharide (LPS, also known as endotoxin). These bacteria are most abundant in the gastrointestinal tract. Gram-negative bacteria are not “bad,” and many are, in fact, beneficial, 2 but too many in the wrong place can be problematic. Endotoxin is best known for its role in sepsis – a rare and extreme, life-threatening inflammatory response to an infection, which is triggered in part by a flood of LPS in the bloodstream called “endotoxemia”. 3 However, research also suggests that moderate levels of LPS in the blood, far less than in sepsis but more than in healthy conditions, may contribute to the chronic inflammation associated with obesity, diabetes, depression, thyroid disease, low testosterone, and more. One of the strongest associations may be with metabolic syndrome. This syndrome is a cluster of at least 3 of the 5 criteria, including: low HDL (high-density lipoprotein), elevated triglycerides, elevated blood pressure, elevated blood glucose (hyperglycemia) and abdominal obesity. ATP III criteria for metabolic syndrome by NCEP (National Cholesterol Education Program) and updates to the criteria are outlined in detail here.
The esteemed research team of Patrice Cani first demonstrated in 2007 that a high-fat diet, which reliably causes weight gain, insulin resistance, and chronic inflammation in mice, leads to a 2-3-fold increase in blood levels of LPS and an increase in LPS-containing bacteria in the gut. 4 They called this “metabolic endotoxemia” to differentiate it from the severe form associated with sepsis. The researchers then found that a direct infusion of LPS into the bloodstream could cause the same weight gain, insulin resistance, and chronic inflammation as a high-fat diet without any change in food intake. Furthermore, they found that eliminating LPS-containing bacteria in the gut substantially reduced these metabolic changes on a high-fat diet. 5 This suggests LPS may play an important role in the relationship between diet and the metabolic syndrome that contributes to cardiovascular risk.
The effects of metabolic endotoxemia
Observational studies in humans support these findings, and as well as correlations with many more conditions. In a large population study in Finland, higher levels of LPS correlated with a harmful cholesterol profile and poor blood sugar control, especially among those with metabolic syndrome. 6 In men, higher exposure to LPS also correlates with lower testosterone, greater obesity rates, and higher inflammatory markers. 7 Twelve of 14 studies have found that both type 1 and type 2 diabetes are associated with higher LPS concentrations in blood. 8 Primary hypothyroidism, or an underactive thyroid gland, is associated with increased LPS, and fecal transplant from hypothyroid humans to healthy mice similarly reduced T4 thyroid hormone level, a hormone produced by the thyroid gland. 9 Metabolic endotoxemia may also play a role in liver and kidney disease, 10,11 and LPS can even increase cortisol, the stress hormone. 12
LPS may also play a role in depression and hostile behavior. Greater degrees of hostile language expressed during discussion of a marital disagreement have been associated with significantly higher blood markers of LPS exposure and inflammation. 13 This effect may be mediated by reductions in serotonin, as LPS has been shown to increase serotonin breakdown and removal in mouse models. 14,15
Three factors that can reduce metabolic endotoxemia
There are three important therapeutic strategies to reduce LPS. First is reducing the amount of gram-negative, LPS-containing bacteria in the gut, as Cani’s initial studies elegantly demonstrated. However, as we previously discussed, the goal is not total elimination. Many gram-negative species are beneficial and perhaps even vital to human health. In fact, recent research has found that LPS’s harmful effects are specific to the type of endotoxin, which varies in different gram-negative species – endotoxin from some bacteria is not only benign, but may actually be protective against the damaging effects of harmful LPS. 16
Another important factor is normalizing intestinal permeability. Our recent blog post addressed the importance of maintaining a healthy gut barrier for immune system balance, but the impacts touch nearly every system of the body. LPS binding protein, a stable marker for endotoxin exposure in the bloodstream, correlates consistently with markers of intestinal permeability. 17 This strongly suggests that it’s not just the quantity of LPS in the gut that determines the levels in the blood, but also the integrity of the gut lining. However, targeting intestinal permeability and LPS-containing bacteria are not isolated strategies, as our gut microbes play a central role in regulating the gut barrier.
This takes us to the third strategy: balancing the overall gut microbiome. Certain gut bacteria that have been found to be depleted in individuals with atherosclerosis play a significant role in reducing the amount of LPS that reaches the bloodstream. 18 Atherosclerosis-prone mice treated with these depleted species of bacteria had lower LPS levels in blood and developed fewer and smaller arterial lesions. Indeed, supporting the growth of healthy microbes may lead to novel therapeutic strategies for obesity, metabolic syndrome, depression, hypothyroidism, and the numerous other conditions affected by metabolic endotoxemia.
Many of the “tried and true” lifestyle interventions for metabolic diseases will also contribute to reducing LPS. A weight-loss plan including a low-calorie and low-saturated fat diet, an exercise routine, and a protein and fiber supplement reduced LPS along with abdominal obesity, blood lipids, and insulin resistance in individuals with metabolic syndrome. 19 In another study, merely 14 minutes of high intensity interval training (HIIT) twice per week reduced LPS, metabolic syndrome parameters, and inflammation to a greater extent than 50 minutes of resistance training twice per week. 20 An intermittent fasting regimen, involving a 70% reduction in caloric intake on two non-consecutive days per week with no restriction the other five, reduced fat mass and inflammation while restructuring the microbiome and reducing blood LPS. 21 Three servings per day of either whole grains or whole fruits and vegetables significantly reduced LPS exposure and inflammatory markers. 22
The most reliable tests for LPS used in research, serum LPS and LPS binding protein, are not currently available clinically. However, comprehensive stool tests are often ordered as part of an assessment by functional medicine physicians, in order to assess intestinal permeability and specific microbial species present in the large intestine, in combination with inflammatory markers, blood glucose and insulin level, as well as a comprehensive lipid panel. While these tests are not a substitute for LPS, they may provide clues as to whether LPS may be playing a role in chronic conditions. To optimize metabolic health, an evidence-based, personalized combination of specific gut-directed, as well as lifestyle and nutritional interventions and therapeutic modalities may be used to effectively support a healthy metabolism, normal blood glucose, reduce inflammation and reduce the risk of cardiovascular disease.
For more information on testing to assess cardiovascular risk, metabolic health and the gut microbiome, you may reach out to our practice at 646.627.8000, email our practice manager Bridget Shaffo at Bridget@drbojana.com.
References:
1. Qin J, Li R, Raes J, et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature. 2010;464(7285):59-65. doi:10.1038/nature08821. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3779803/
2. Sonnenborn U. Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 2016;363(19):fnw212. doi:10.1093/femsle/fnw212. https://pubmed.ncbi.nlm.nih.gov/27619890/
3. Buttenschoen K, Radermacher P, Bracht H. Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbecks Arch Surg. 2010;395(6):597-605. doi:10.1007/s00423-010-0658-6. https://pubmed.ncbi.nlm.nih.gov/20582603/
4. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-1772. doi:10.2337/db06-1491. https://pubmed.ncbi.nlm.nih.gov/17456850/
5. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470-1481. doi:10.2337/db07-1403. https://pubmed.ncbi.nlm.nih.gov/18305141/
6. Määttä AM, Salminen A, Pietiäinen M, et al. Endotoxemia is associated with an adverse metabolic profile. Innate Immun. 2021;27(1):3-14. doi:10.1177/1753425920971702. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7780360/
7. Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314(3):E206-E213. doi:10.1152/ajpendo.00279.2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5899218/
8. Gomes JMG, Costa J de A, Alfenas R de CG. Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism. 2017;68:133-144. doi:10.1016/j.metabol.2016.12.009. https://pubmed.ncbi.nlm.nih.gov/28183445/
9. Su X, Zhao Y, Li Y, Ma S, Wang Z. Gut dysbiosis is associated with primary hypothyroidism with interaction on gut-thyroid axis. Clin Sci Lond Engl 1979. 2020;134(12):1521-1535. doi:10.1042/CS20200475. https://pubmed.ncbi.nlm.nih.gov/32519746/
10. Chastre A, Bélanger M, Nguyen BN, Butterworth RF. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int Off J Int Assoc Study Liver. 2014;34(3):353-361. doi:10.1111/liv.12252. https://pubmed.ncbi.nlm.nih.gov/23910048/
11. Liu X, Lu J, Liao Y, et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed Pharmacother Biomedecine Pharmacother. 2019;117:109070. doi:10.1016/j.biopha.2019.109070. https://pubmed.ncbi.nlm.nih.gov/31176164/
12. Bach E, Møller AB, Jørgensen JOL, et al. Stress hormone release is a key component of the metabolic response to lipopolysaccharide: studies in hypopituitary and healthy subjects. Eur J Endocrinol. 2016;175(5):455-465. doi:10.1530/EJE-16-0444. https://pubmed.ncbi.nlm.nih.gov/27562403/
13. Kiecolt-Glaser JK, Wilson SJ, Bailey ML, et al. Marital Distress, Depression, and a Leaky Gut: Translocation of Bacterial Endotoxin as a Pathway to Inflammation. Psychoneuroendocrinology. 2018;98:52-60. doi:10.1016/j.psyneuen.2018.08.007. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6260591/
14. Korte-Bouws GAH, van Heesch F, Westphal KGC, et al. Bacterial Lipopolysaccharide Increases Serotonin Metabolism in Both Medial Prefrontal Cortex and Nucleus Accumbens in Male Wild Type Rats, but Not in Serotonin Transporter Knockout Rats. Pharmaceuticals. 2018;11(3):66. doi:10.3390/ph11030066. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6160917/
15. Zhao R, Wang S, Huang Z, et al. Lipopolysaccharide-induced serotonin transporter up- regulation involves PKG-I and p38MAPK activation partially through A3 adenosine receptor. Biosci Trends. 2015;9(6):367-376. doi:10.5582/bst.2015.01168. https://www.jstage.jst.go.jp/article/bst/9/6/9_2015.01168/_article
16. Anhê FF, Barra NG, Cavallari JF, Henriksbo BD, Schertzer JD. Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep. 2021;36(11):109691. doi:10.1016/j.celrep.2021.109691. https://pubmed.ncbi.nlm.nih.gov/34525353/
17. Seethaler B, Basrai M, Neyrinck AM, et al. Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol Gastrointest Liver Physiol. 2021;321(1):G11- G17. doi:10.1152/ajpgi.00113.2021. https://journals.physiology.org/doi/full/10.1152/ajpgi.00113.2021
18. Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.033714
19. Guevara‐Cruz M, Flores‐López AG, Aguilar‐López M, et al. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2019;8(17):e012401. doi:10.1161/JAHA.119.012401. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6755842/
20. Reljic D, Dieterich W, Herrmann HJ, Neurath MF, Zopf Y. “HIIT the Inflammation”: Comparative Effects of Low-Volume Interval Training and Resistance Exercises on Inflammatory Indices in Obese Metabolic Syndrome Patients Undergoing Caloric Restriction. Nutrients. 2022;14(10):1996. doi:10.3390/nu14101996. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9145085/
21. Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J Clin Endocrinol Metab. 2021;106(1):64-79. doi:10.1210/clinem/dgaa644. https://pubmed.ncbi.nlm.nih.gov/33017844/
22. Kopf JC, Suhr MJ, Clarke J, et al. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: a randomized controlled trial. Nutr J. 2018;17(1):72. doi:10.1186/s12937-018-0381-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6066923/